UNISTAT® bioassay analysis software has been integrated for use with ProtoCOL 3 colony counting and inhibition zone measuring system. The statistical module allows scientists a quick, easy method of analysing their ProtoCOL 3 data to generate accurate antibiotic and vaccine potency results.
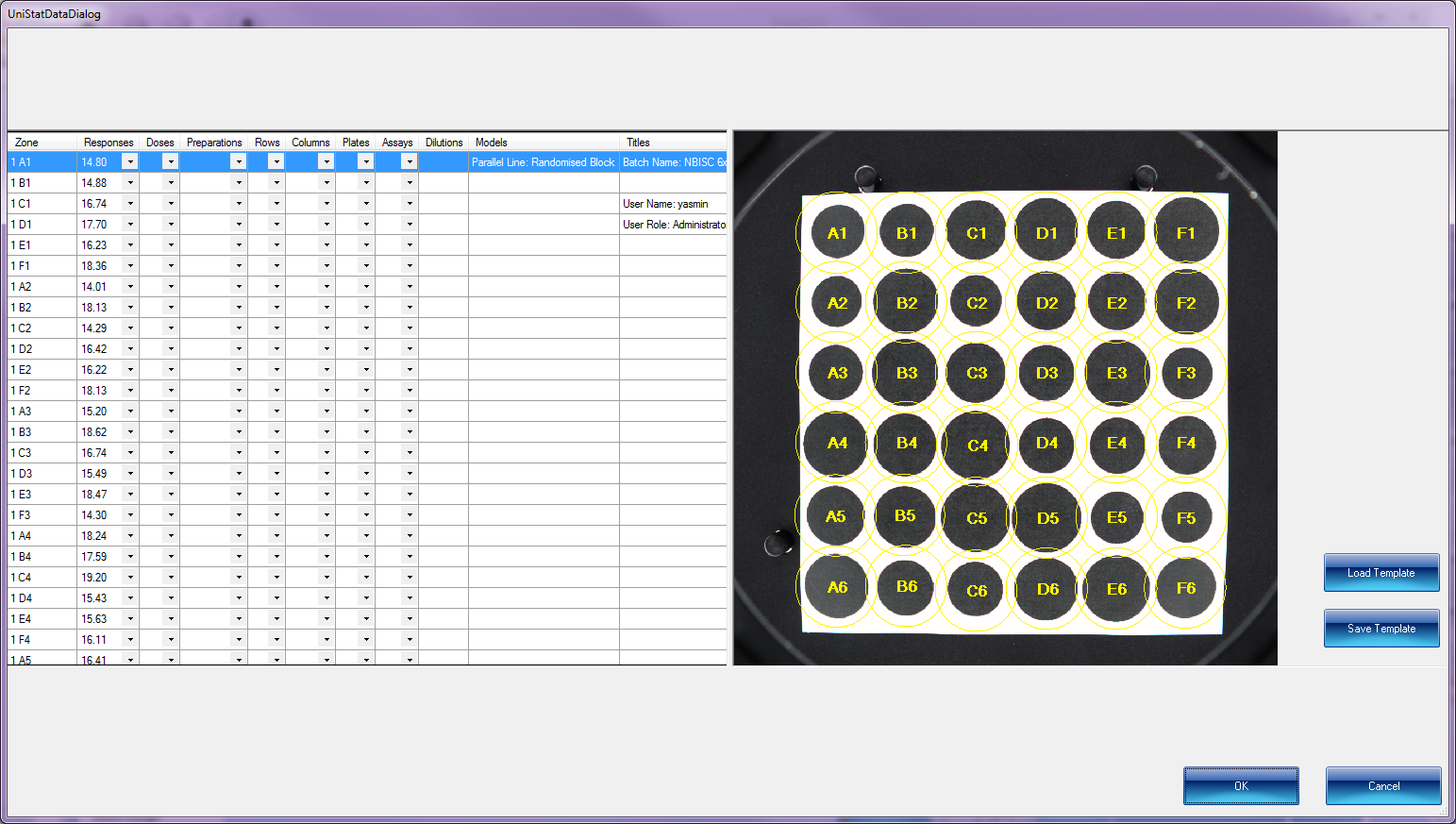
With just one click of the new UNISTAT bioassay statistics module, microbiologists can export inhibition zone measurement and colony count data from ProtoCOL 3 directly to UNISTAT.
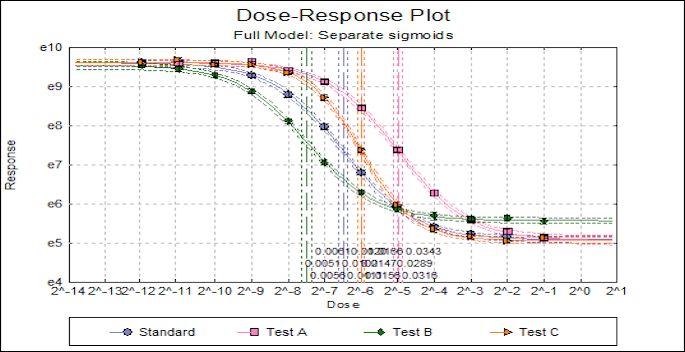
Statistical analysis of data from biological dilution assays or vaccine/antibiotic potency assays: Potency calculations can be performed employing parallel line, slope ratio, quantal response or four-parameter logistic model, complete with fiducial confidence intervals, validity tests, potency, ED50, Spearman-Karber method and graphical representations.
- Parallel Line Assay: Completely Randomised Design, Randomised Block Design, Latin Squares Design, Crossover Design
- Slope Ratio Assay: Blanks, Plate Effects
- Quantal Response Assay: Logit, Probit, Gompit, Loglog link functions
- Four-Parameter Logistic Model: European Pharmacopoeia (EP), Full Model (USP), Reduced Model (USP)
- Combination of Assays: EP, USP
- Cylinder-Plate (5+1) Assay
UNISTAT is available as an optional software module for ProtoCOL 3.