Single Radial Immunodiffusion (SRID)
What is SRID?
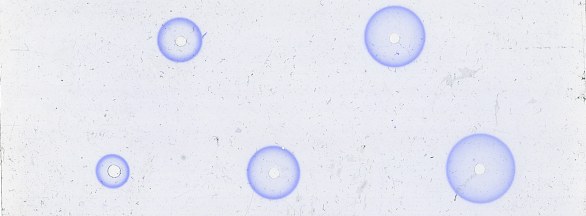
Single Radial Immunodiffusion is a technique used extensively for the quantitative estimation of antigens. Antibody of known specificity is distributed evenly in an agar gel and a sample containing the antigen of interest is placed in a well within the gel. Antigen then diffuses radially from the well and a precipitin ring forms at the point of antibody-antigen equilibrium. Antigen concentrations are determined by measuring the diameter of precipitin rings and extrapolating using standard curves.
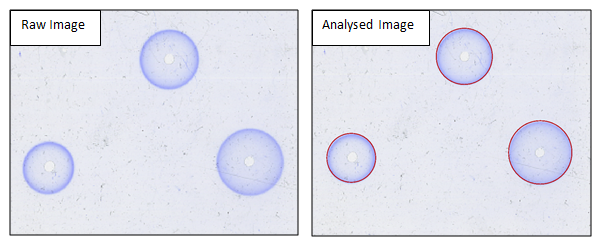
Figure 1. Side by side comparison of raw and analysed SRID plate images in the ProtoCOL 3 software
What does Synbiosis offer?
Zone measurement software comes as standard with the ProtoCOL 3 systems. This ‘Inhibition Zone’ module enables the researcher to analyse SRID plates in a single zone, ring or grid layout which makes it suitable for various styles of testing. Zone diameter and area measurements are provided in millimetres. The zone measurement module is also featured in the IVD-certified ChromaZona, which is specially designed for clinical applications.
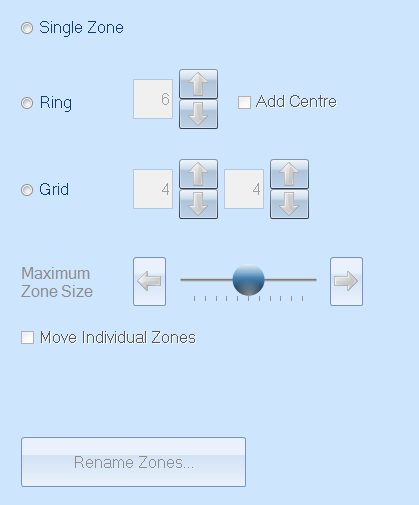
Figure 2. Manual zone classification step in the ProtoCOL 3 software
Zone classification is performed by indicating the diameter of wells and selecting typical colours of the disc/well, zone and background. Once the initial setup steps are completed, SRID plates can be analysed at the push of a button.
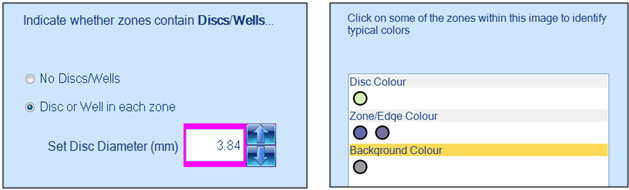
Figure 3. Classification windows in the ProtoCOL 3 software for setting disc/well size (left) and selecting colours of disc, zone and background (right)
SRID results can be analysed using UNISTAT, our 3rd party statistics module. Study templates can be designed in ProtoCOL 3 that will transfer seamlessly to UNISTAT enabling scientists to perform statistical analysis on the results of their study

Figure 4. Dialog box in the ProtoCOL 3 software for the incorporation of UNISTAT statistics package to analyse the SRID results.
Compliance
The ProtoCOL 3 system is fully CFR 21 Part 11 compliant with a full audit trail, user access levels and LIMS connection. Results produced from SRID assays are compliant with GCP (Good Clinical Practice) guidelines which can be presented to regulatory authorities such as the European Medicines Control Agency (EMEA) and Food and Drug Administration (FDA).
The zone measurement results are automatically transferred into a table for storage in a secure database. The database is password protected ensuring that batches of results cannot be deleted. Additionally, any changes are recorded with a coded flag next to the revised result. Every detail of the sample including images of the SRID plates, system configuration, user details, date and time are recorded in a professional report (Excel, Open Office or PDF).
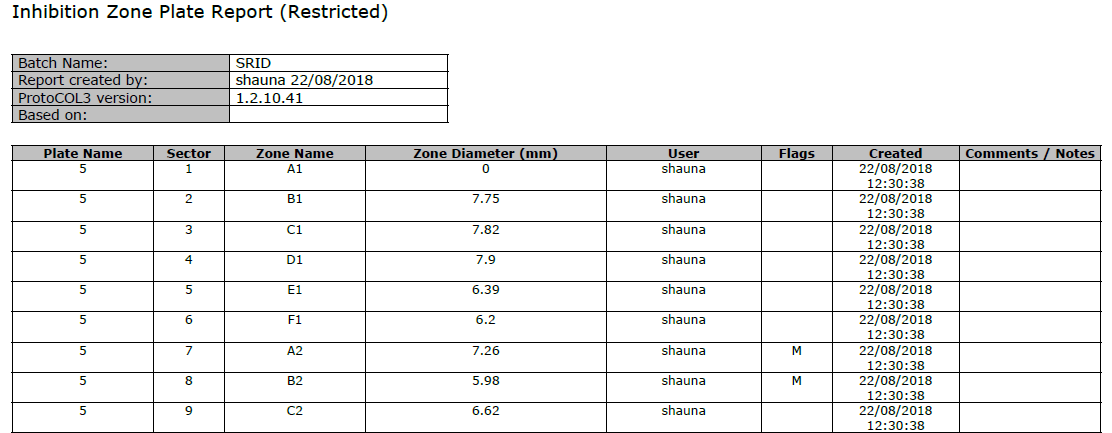
Figure 5. Example of a PDF results report generated in ProtoCOL 3
