Antibiotic Susceptibility Testing (AST)
What is Antibiotic Susceptibility Testing?
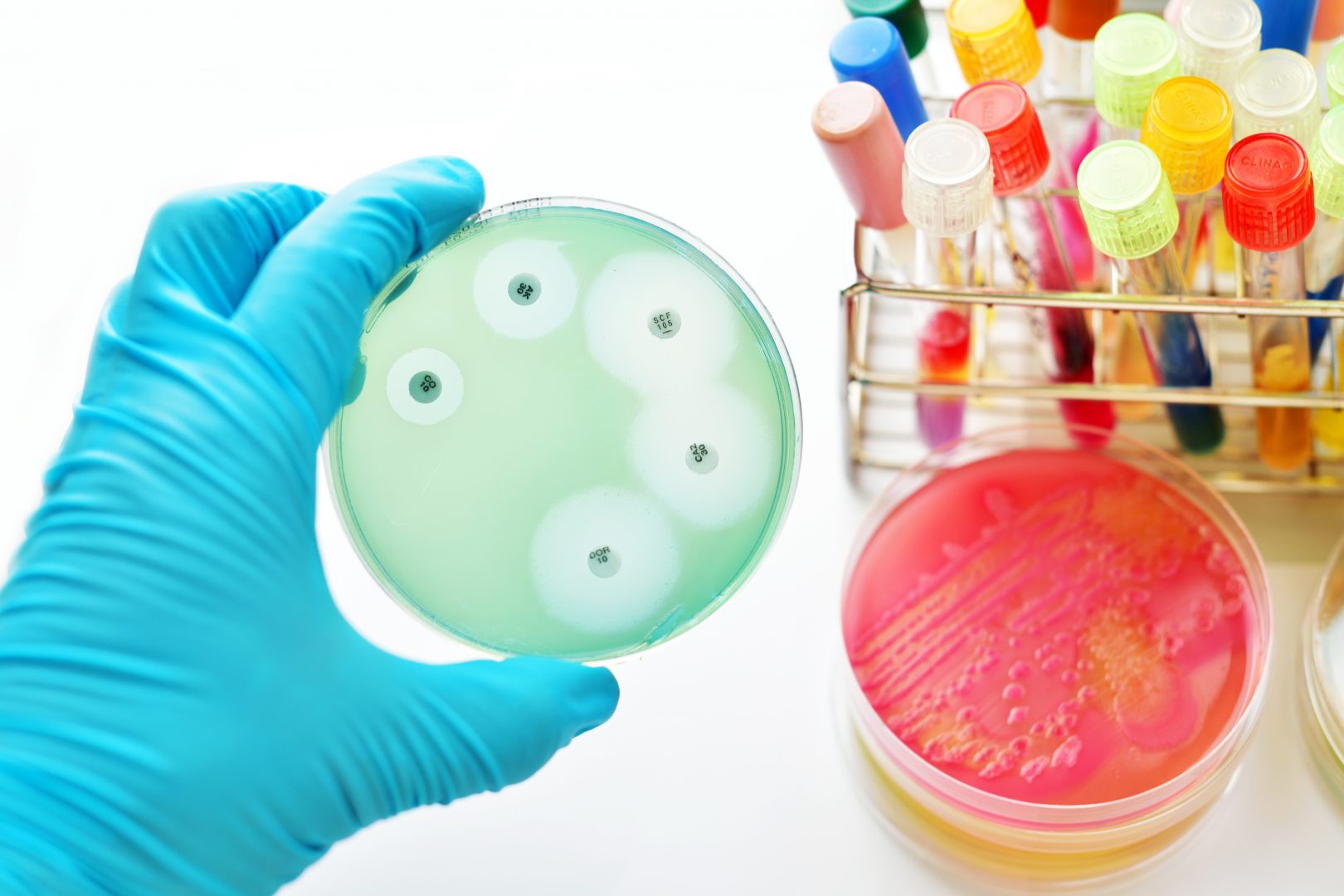
Antibiotic susceptibility testing, or AST, is a widely-used method of evaluating antibiotic resistance and determining patient treatment plans in clinical settings. There are a number of different methods of AST such as agar dilution, broth dilution and disc diffusion assays. The disc diffusion or ‘Kirby-Bauer’ method involves spreading bacteria on an agar plate and placing paper discs impregnated with antibiotic on the plate. After incubation, the growth of bacteria is observed. Areas around the antibiotic disc where no bacterial growth can be seen are known as ‘zones of inhibition’. These zones show that an antibiotic has been successful in stopping bacterial growth or killing the bacteria. By measuring the diameter of these zones, we can compare the efficacy of antibiotics and monitor antimicrobial resistance.
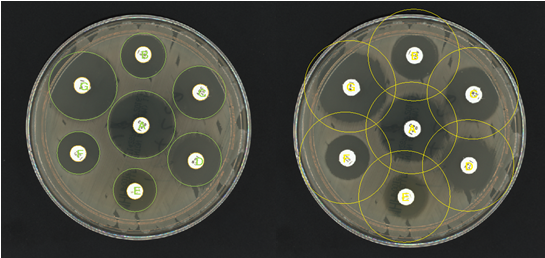
Figure 1. Before and after images of an AST plate analysed in ProtoCOL 3
Benefits of automation
AST can be a time-consuming process so many laboratories look to automation to speed-up testing and increase repeatability. The eAST software not only measures inhibition zone diameters but can compare the data to the most up-to-date European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Clinical Laboratory Standards Institute (CLSI) guidelines. This provides clinicians and researchers with rapid SIR (Susceptible, Intermediate and Resistant) results and access to expert rules, to aid in their studies. Within the eAST software, zones can be classified in ring, grid or single zone formats. Once this format has been chosen and the software has been told what colours to look for, plates can be analysed at the push of a button.
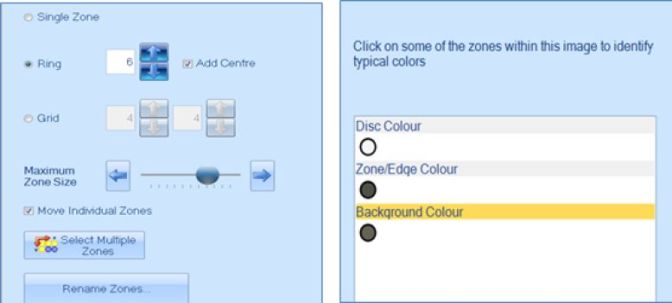
Figure 2. Zone and disc classification step in the ProtoCOL 3 software
An exciting feature of the eAST software is that plates can be analysed based on EUCAST/CLSI guidelines, manually-entered breakpoints or a combination of both. This enables researchers to create a database of their own antibiotic information whilst still having the EUCAST/CLSI database at their disposal.

Figure 3. Dialog window in ProtoCOL 3 software for choosing breakpoints and expert rules and specifying between EUCAST, CLSI and manual breakpoints.
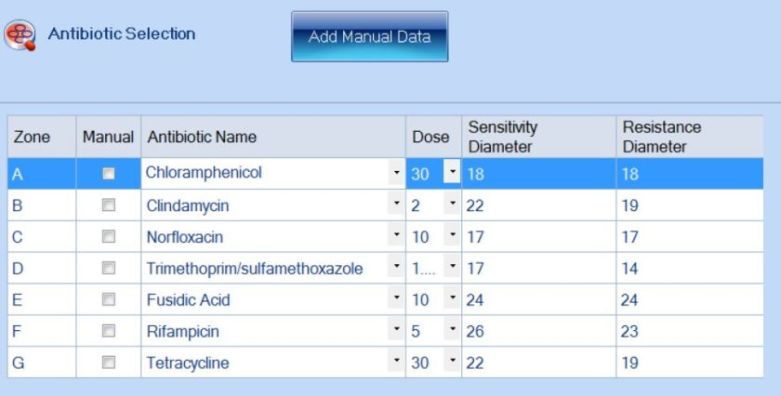
Figure 4. Addition of antibiotic information in the ProtoCOL 3 software
Data is stored in a secure SQL database for traceability and can be transferred to a spreadsheet (Excel/Open Office), PDF or LIMS. This eliminates keying and data transfer errors, producing fully traceable results which are consistent from microbiologist to microbiologist.
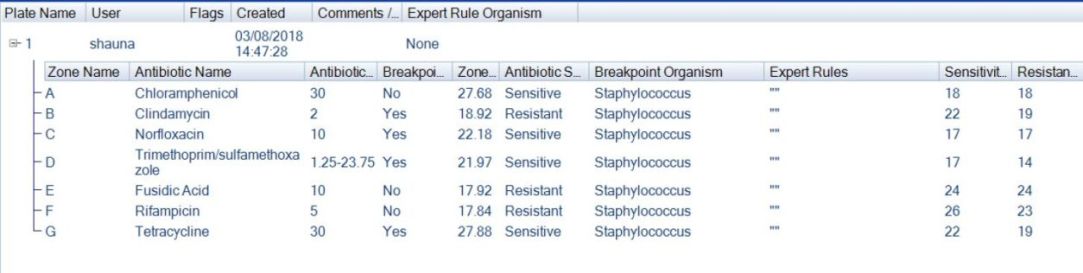
Figure 5. Example of AST plate results tab in ProtoCOL 3 software
The software is GLP and GDP compliant and can be used in a 21 CFR Part 11 environment. It includes user access levels and a full audit trail with user log-in and log-out records. These archived results are suitable for generating reports for audit by regulatory authorities and can also be used in hospitals wanting to identify and monitor incidence of microbial resistance, making the new eAST software suitable for use in highly regulated microbiology laboratories.

Figure 6. Setting different user permissions between users, advanced users and administrators in the ProtoCOL 3 software
The ProtoCOL 3 user permissions allow Administrators, such as laboratory managers, to specify what actions each level of technician can perform within the software. This eliminates the risk of accidental changes or deletions being made by technicians.
The eAST software is included in the IVD-registered ChromaZona as standard and is available as an optional module for ProtoCOL 3 systems. Synbiosis offers free bi-annual software upgrades which ensure that labs have access to the newest features and most up-to-date EUCAST and CLSI guidelines.
